Nobel Prize-winning former molecular biologist Joshua Lederberg coined the term ‘microbiome’ in 2001 to signify the ecological community of all living organisms that share our body space.
Although technically, the human microbiome was defined as the complete genome of all microorganisms in and on the human body, the term is now usually used synonymously to refer to the community of all microorganisms in this habitat.
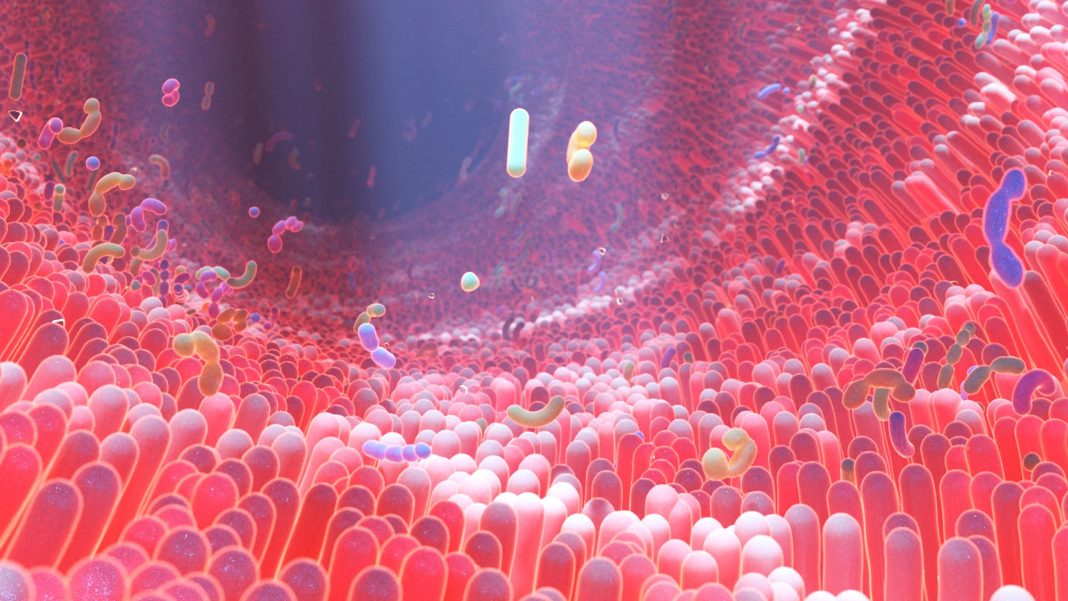
Our human body is a complex ecosystem that has trillions of microbes, without which we would struggle to digest and break down essential nutrients.
These microbes receive signals from the body concerning our state of health, and keep our immune system in check by protecting against infection. They inhabit all parts of our body that are exposed to the environment, including our skin, mouth, urogenital tract, airways and gut.
The tasks of the gut microbiome include metabolic functions, such as the synthesis of vitamins, decomposition of chemicals and nutrients, and support of fat metabolism, but also the interplay with the immune system, such as the outcompeting of pathogens, and the stimulation and maturation of the immune system.
Our immune system is engaged in a constant, highly sensitive balancing act between states of aggression and tolerance. It can manage this balancing act only with the help of microbiota – all the living members forming the microbiome. The microbiota teaches the immune system which cells to fight and which cells to leave alone. If these bacteria are missing, our body overreacts to all kinds of cellular material we pick up, resulting in allergies and asthma.
Our bodies resemble ecosystems as diverse as planets, with each body site harbouring a specific ecological community with specific tools. A perturbation of one of these communities, also called dysbiosis, makes us feel very sick – and we’re only just beginning to discover how devastating this is.
Removing our beneficial bacteria makes us vulnerable to pathogenic bacteria, which move in on a wiped-out ecosystem where they can then settle and make us sick.
However, the microbiome of healthy people eventually recovers within a year, although immune-deficient people struggle to regain their healthy microbiome, which results in a vicious circle of the need for more antibiotics to be taken.
Environmental factors, including unbalanced diets and the widespread use of antibiotics, among others, reduce the diversity of our microbiome.
The American Gut Project
After five years of research, in May 2018, scientists at the University of California San Diego and collaborators initiated the American Gut Project, analysing more than 15,000 microbiome samples from more than 11,000 participants from mostly the US, the UK and Australia, but also from 42 other countries and territories.
The results so far include:
- The more different plant types a person eats, the higher the microbial diversity of the gut. Persons who ate 30 or more different plant types a week had microbiomes that were more diverse than those of people who ate only 10 plant types or less per week.
- The administration of antibiotics lowered the microbial diversity of the gut, as expected. One surprising result was that the diversity of the molecules found in people who had taken antibiotics was much higher than in people who hadn´t taken antibiotics for more than a year. These molecules found seemed to be linked to exposure to antibiotics.
- Another unexpected result was the detection of agricultural antibiotics in people who claimed that they hadn’t taken antibiotics in the year before their sample collection. This means that with the meat we eat, we still might take up antibiotics, which harm our microbiome.
- A comparison of two distinct Western populations revealed significant differences in the diversity of the samples: people in the UK seemed to have a higher microbial diversity than people from the US.
- The researchers discovered a link between the composition of the microbiome and people with depression. Although taken from two different sides of the Atlantic Ocean, the samples proved to be consistent in the US and UK populations. This shows that the microbiome and disease strongly influence each other, independent of the environment the person lives in.
The role of pharmacy assistants
In an interview published on the Medibank.com.au site, Brinley Hosking, Chief Pharmacist at Amcal, advises that customers needing help and advice on putting practices into place to keep their gut health strong and healthy can find at their local pharmacy a range of health-related services and management for many health-related issues, including those that may trigger gut problems. He points to pharmacy services such as type 2 diabetes screenings, support for quitting smoking, blood pressure checks, allergy support and more.
Pharmacy assistants can answer most questions about the medications customers are currently taking, and if they’re not unwell but want to be as healthy as possible, pharmacists can also provide general lifestyle advice and answer questions about specific products they may need to take. Products sold at Australian pharmacies include vitamins and supplements, non-prescription medicines for minor ailments (such as cuts, cough, colds, and minor pains), and prescription medicines for chronic disease and serious conditions.
Pharmacy assistants can suggest alternative lifestyle practices to consider, to keep gut issues under control. For those with medically diagnosed health issues, the community pharmacy can work closely with their doctor, and monitor medications of those prone to infection. They can recommend alternative medications or products while educating on why certain things work and don’t work.
This feature was originally published in the July issue of Retail Pharmacy Assistants e-magazine.
To read the feature in full as it appears in the e-magazine, visit: rpassistants.com.au/retail-pharmacy-assistants-july-2022